Amino Acids and Proteins
Amino acids are the building blocks of proteins, which are essential macromolecules in all living organisms. Proteins perform a wide range of functions, including catalyzing biochemical reactions, providing structural support, and regulating cellular processes. In this section, we will explore the structure and function of amino acids and proteins, as well as their roles in biological systems.
Table of Contents
Amino Acids
Proteins are the most diverse class of biomolecules, and they serve many biological functions. Amylase, lipase, and pepsin are all digestive enzymes that catalyze the breakdown of carbohydrates, lipids, and proteins, respectively. Hemoglobin is a protein that transports oxygen in the blood, while collagen provides structural support to tissues and organs. Insulin and glucagon are hormones that regulate blood sugar levels, and antibodies are proteins that help the immune system fight infections. Proteins are polymers made of monomeric units called amino acids.
An amino acid has the general structure
where
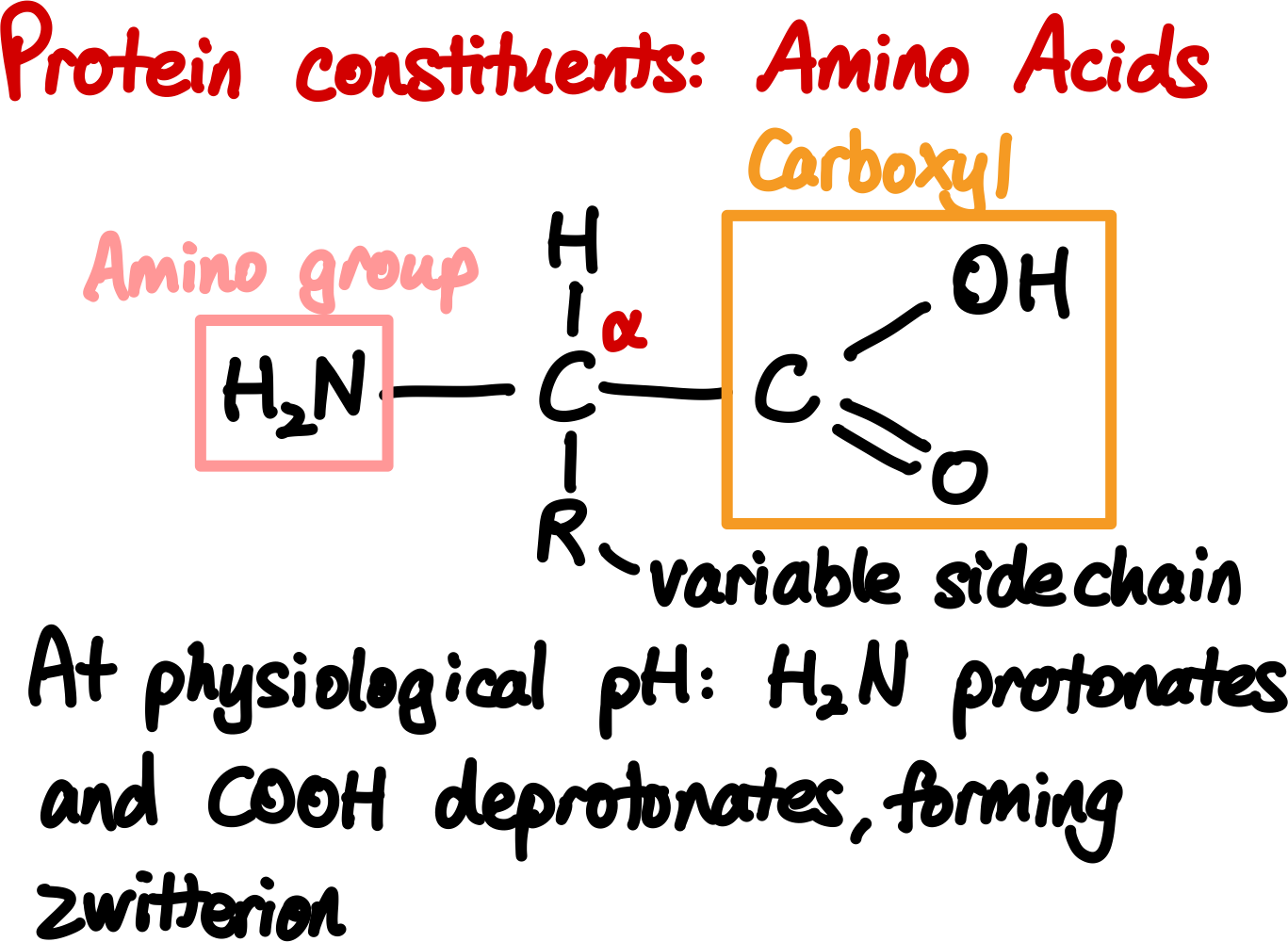
There are a few important properties of amino acids that we should note.
First, with the exception of glycine (where
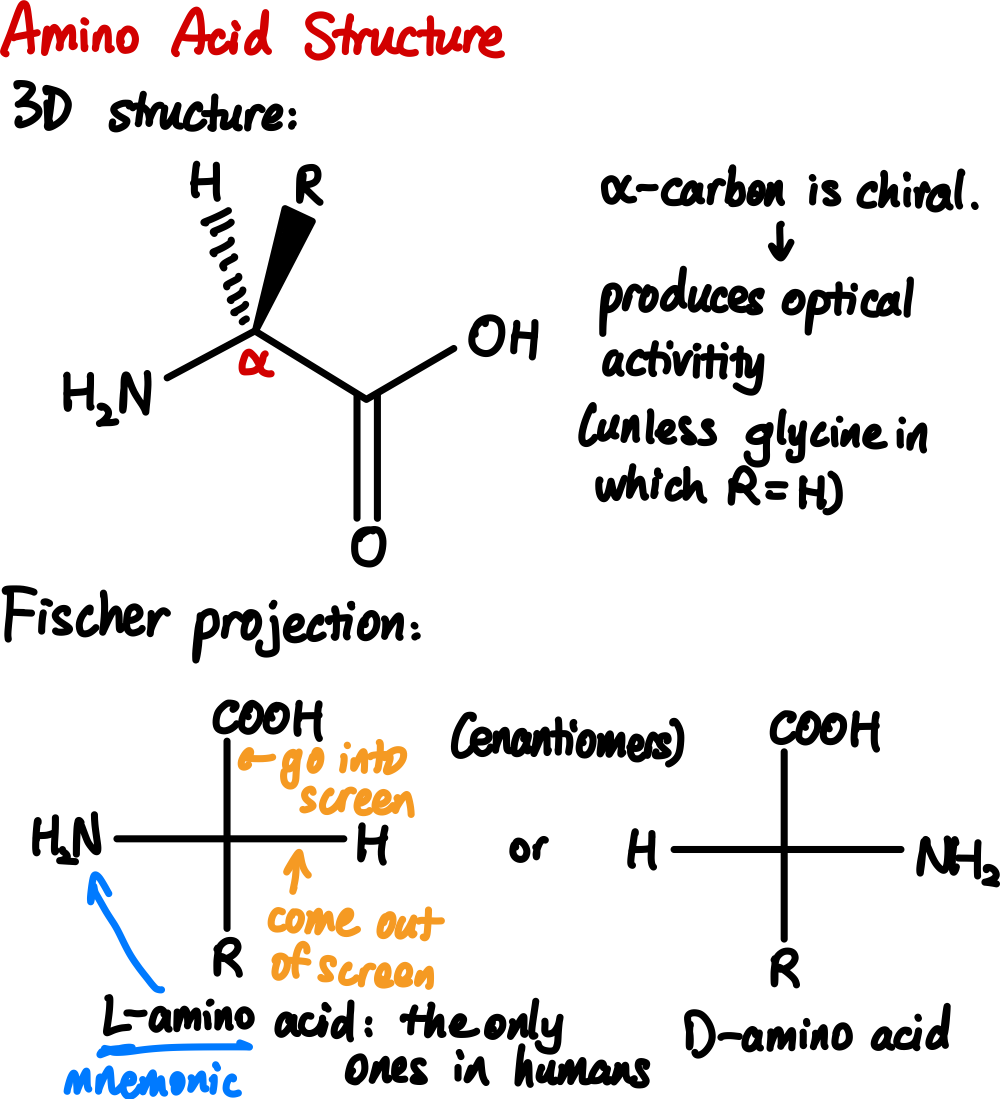
Second, if you use the standard steric number system, you would guess that the nitrogen is tetrahedral (as it has four substituents), but it is actually trigonal planar. This is because the planar structure allows for conjugation between the lone pair of electrons on the nitrogen and the carbonyl oxygen, which stabilizes the molecule.
Third, amino acids can exist in different ionization states depending on the pH of the solution.
At low pH, the amino group is protonated (
Peptides
Amino acids can be linked together to form peptides, which are short chains of amino acids. A peptide bond is formed between the carboxyl group of one amino acid and the amino group of another amino acid, resulting in the release of a water molecule (a condensation reaction). The reaction is a dehydration synthesis (or condensation) reaction, and it is classified as a nucleophilic addition-elimination reaction. The resulting peptide bond is a covalent bond that links the two amino acids together.
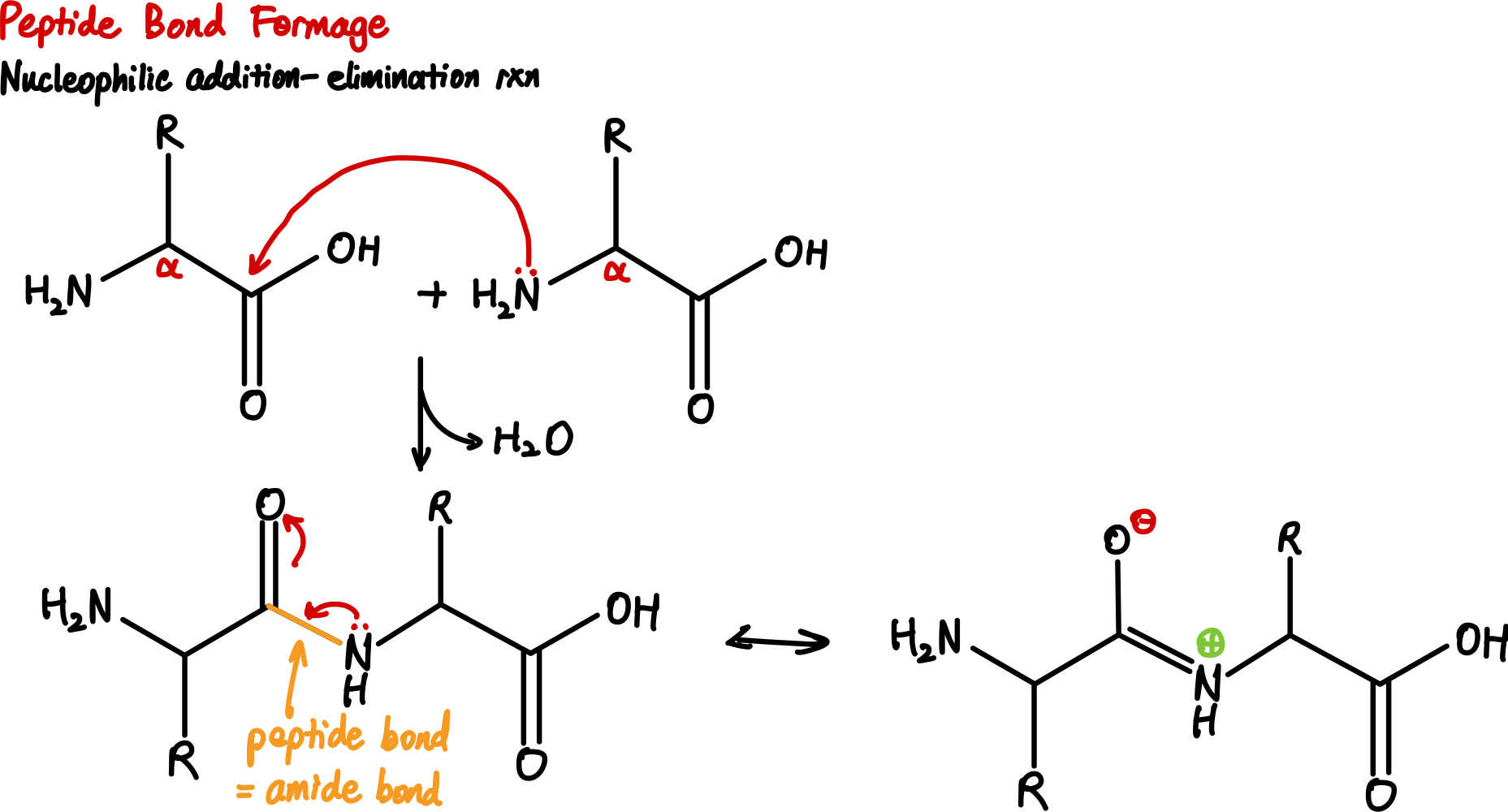
Peptide bonds are rigid and planar due to the partial double bond character of the bond, which is a result of resonance between the carbonyl oxygen and the nitrogen. This rigidity restricts the rotation around the peptide bond, resulting in a fixed conformation of the peptide. This will be important when we discuss protein structure later.
We can also cleave peptide bonds in two ways. First, we can use hydrolysis, which is the reverse of the condensation reaction. It requires heat and a strong acid or base to break the peptide bond. Hydrolysis is non-specific; it will cleave any peptide bond in the protein without regard to the amino acid sequence.